



 epilepsy (a disorder of the central nervous system characterized by loss of consciousness and convulsions)
epilepsy (a disorder of the central nervous system characterized by loss of consciousness and convulsions)There are many types of seizures, depending primarily on what part of the brain is involved. The term epilepsy says nothing about the type of seizure or cause of the seizure, only that the seizures happen again and again. A stricter definition of the term requires that the seizures have no known underlying cause. This may also be called primary or idiopathic epilepsyEpisodes of abnormal electrical activity within the brain result in seizures. The specific area of the brain affected by the abnormal electrical activity may result in a particular type of seizure.
If all areas of the brain are affected by the abnormal electrical activity, a generalized seizure may result. This means that consciousness is lost or impaired. Often all the person's arms and legs stiffen and then jerk rhythmically.
One seizure type may evolve into another during the course of the seizure. For example, a seizure may start as a partial, or focal, seizure, involving the face or arm. Then the muscular activity spreads to other areas of the body. In this way, the seizure becomes generalized.
Seizures caused by high fevers in children are not considered epilepsy. Also see children's seizures
The age at which the seizures begin
The causes of the seizures
Whether the seizures are inherited
The part of the brain involved
Factors that provoke seizures
How severe and how frequent the seizures are
A pattern of seizures by time of day
Certain patterns on the EEG, during seizures and between seizures
Other disorders in addition to seizures
The prospects for recovery or worsening
Partial seizure
A simple partial seizure will often be a precursor to a larger seizure such as a complex partial seizure, or a tonic-clonic seizure. When this is the case, the simple partial seizure is usually called an aura.
Partial seizures are common in temporal lobe epilepsy
Seizures occur when the neurons in the brain suddenly increase activity, causing an electrical storm that can overwhelm the brain. This can result in various symptoms depending on the area of the brain affected. People who have a generalized seizure usually experience symptoms that affect their entire body, such as whole-body muscle contractions or a loss of consciousness.
Generalized seizures may be caused by chronic underlying medical conditions that may require treatment (e.g., epilepsy). Many generalized seizures have no known cause, making them difficult to prevent. In cases where the underlying cause is unknown, seizures can sometimes be controlled with medication.
Generalized seizures can rarely be treated with brain surgery because the abnormal neuron activity occurs in the entire brain. However, people with recurrent generalized seizures that are poorly controlled by medication may be suitable for a type of treatment called vagus nerve stimulation.
There are certain things bystanders can do (and not do) to prevent additional harm to a person having a generalized seizure. For example, nothing should be placed in a person’s mouth during a seizure, and restraint should not be used. It is recommended that bystanders clear the area of furniture and objects that may cause injury to the person having a seizure. Also, the person having the seizure should be gently rolled onto his or her side to prevent choking on vomit or mucus
Center for Treatment of Epilepsy and Migraine, Kielecka 25, 31-523 Kraków, 2Department of Neurology,
Neuropsychiatric Care Unit, Grunwaldzka 47, 25-736 Kielce, 3Department of Pathophysiology, Skubiszewski Medical
University, Jaczewskiego 8, 20-090 Lublin, 4Isotope Laboratory, Institute of Agricultural Medicine, Jaczewskiego 2,
20-950 Lublin, Poland
via a specific subunit of voltage-dependent calcium channels. Conventional antiepileptics generally inhibit sodium currents (carbamazepine, phenobarbital, phenytoin, valproate) or enhance GABA-ergic inhibition (benzodiazepines, phenobarbital, valproate). Ethosuximide, mainly controlling absences, reduces calcium currents via T-type calcium channels. Novel antiepileptic drugs, mainly associated with an inhibition of voltage-dependent
sodium channels are lamotrigine and oxcarbazepine. Since glutamate-mediated excitation is involved in the generation of seizure activity, some antiepileptics are targeting glutamatergic receptors – for instance, felbamate, phenobarbital, and topiramate. Besides, they also inhibit sodium currents. Zonisamide, apparently sharing this common mechanism, also reduces the concentration of free radicals. Novel antiepileptic drugs are better tolerated by epileptic patients and practically are devoid of important pharmacokinetic drug interactions.
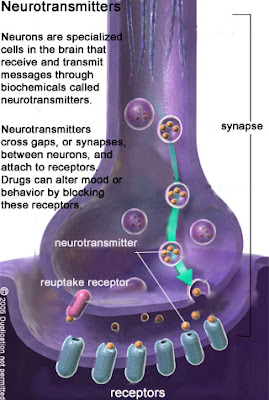
called GABAA and GABAC receptors are distinguished - metabotropic ones linked to the cascade of second intraneuronal messengers are GABAB receptors [4,5].
GABAA receptor complex consists of a number of binding sites for GABA itself, benzodiazepines, barbiturates, ethanol and picrotoxin which is a chloride channel blocker. When GABA binds to its recognition site on the GABAA receptor complex, an opening of the chloride channel occurs with the subsequent influx of chloride anions into a neuron, resulting in its hyperpolarization. Benzodiazepine derivatives (f.e.: diazepam, clonazepam) increase the frequency of the channel
openings whilst barbiturates (f.e.: phenobarbital) prolong the opening time of the channel. Both, benzodiazepines and barbiturates also enhance the affinity of GABAA receptors for
the neurotransmitter [4,5]. In contrast, binding GABA to the GABAB receptors results in the activation of phospholipase A-2 and the following synthesis of arachidonic acid fromphospholipids. Arachidonic acid via regulatory Gi proteins is likely to modulate the activity of adenyl cyclase and cyclic AMP levels. Through the GABAB receptors GABA affects
the release of other important for the neuronal activity neurotransmitters. GABAC receptors are mainly encountered in the retina and their physiological significance is a matter of dispute.
metabolism: aminooxyacetic acid, g -acetylenic-GABA, g - vinyl-GABA or direct agonists, for example - muscimol. Actually, these substances were found to exert anticonvulsant
effects in a variety of experimental models of epilepsy. The initial enthusiasm was, however, not fully justified it soon would come out that muscimol displayed a proconvulsant activity in primates and humans [7]. This was understood in terms of an undesired effects of the diffuse
stimulation of GABAA receptors within the brain. Consequently, the subsequent search for GABA-ergic agents as potential antiepileptic drugs would focus on substances
indirectly enhancing GABA functions - via inhibition of its metabolism or reduction of its neuronal uptake. This strategy led to the discovery of potent anticonvulsant substances -
some of them are nowadays potent antiepileptic drugs
INHIBITION
vigabatrin seem to express their anticonvulsant activity mainly through the GABA-ergic system. Other novel antiepileptics associated with GABA-mediated inhibition, which also share additional mechanisms of action, are: felbamate, gabapentin, and topiramate. Tiagabine and vigabatrin, and to a certain degree - gabapentin, may be considered as drugs whose development was associated with so called GABA hypothesis of epilepsy [13]. Vigabatrin is an irreversible inhibitor of GABA transaminase and its administration in animals or humans results in the 3-fold increase in synaptic GABA level [14-16]. Tiagabine inhibits neuronal and glial GABA uptake, leading thus to the enhancement and prolongation of GABA synaptic events [16,17]. The anticonvulsant activity of inhibitors of GABA uptake in various models of experimental epilepsy was shown much earlier but these substances did not cross the blood-brain barrier. This certainly disqualified their possible use as antiepileptic drugs [18]. Probably, as already mentioned, tiagabine and vigabatrin possess mechanisms of action closely related to GABA-mediated events in the synaptic cleft, in contrast to conventional and some novel antiepileptics which may block voltage-dependent sodium and calcium channels and impair glutamate-induced excitation. For instance, sodium channels are blocked by a variety of antiepileptic drugs, including benzodiazepines (at high concentrations), carbamazepine, felbamate, lamotrigine, oxcarbazepine, phenytoin, phenobarbital, topiramate, and valproate. Ethosuximide or zonisamide mainly affect T-type calcium channels, and felbamate, phenobarbital, and topiramate inhibit glutamate excitation [19]. Interestingly, gabapentin, a cyclic analogue of GABA, was designed as a GABA agonist easily passing the blood-brain barrier. However, no receptor activity of gabapentin was detected on the GABAA receptor complex, only increased GABA turnover being found in some rat brain regions [20,21]. Also, gabapentin was documented to increase GABA level in brains of epileptic patients [15]. It is evident now, that this antiepileptic drug binds to the specific unit of voltagedependent calcium channel and inhibits intraneuronal calcium ion flux from the extraneuronal space [22]. Two novel antiepileptic drugs, topiramate and felbamate, although possessing multiple mechanisms of action (see below), affect GABA-mediated inhibition as well. Specifically, the former seems to potentiate effects of endogenous GABA through a novel binding site on the GABAA receptor complex [21,23]. The latter enhanced GABA-dependent chloride currents in rat hippocampal neurons [24]. However, such effect in vitro was no longer evident in the absence of GABA and, moreover, felbamate was not shown to interact directly with the GABAA receptor complex [25]. Among conventional and novel.





















